Cytokinetics Announces Results of FORTITUDE-ALS, a Phase 2 Clinical Trial of Reldesemtiv in Patients With ALS, Presented at American Academy of Neurology Annual Meeting
Trial Did Not Meet Statistical Significance for Primary Efficacy Analysis
Patients on All Doses of Reldesemtiv Declined Less Than Patients on Placebo
for SVC and ALSFRS-R, With Clinically Meaningful Differences Emerging Over Time
Early Terminations and Serious Adverse Events
Were Balanced Across Treatment Arms
Investor Event & Conference Call on
SOUTH SAN FRANCISCO, Calif.,
FORTITUDE-ALS did not achieve statistical significance for a pre-specified dose-response relationship in its primary endpoint of change from baseline in slow vital capacity (SVC) after 12 weeks of dosing (p=0.11). Similar analyses of ALSFRS-R and slope of the Muscle Strength Mega-Score yielded p-values of 0.09 and 0.31, respectively. However, patients on all dose groups of reldesemtiv declined less than patients on placebo for SVC and ALSFRS-R, with larger and clinically meaningful differences emerging over time.
While the dose-response analyses for the primary and secondary endpoints did not achieve statistical significance at the level of 0.05, in a post-hoc analysis pooling the doses together, patients who received reldesemtiv in FORTITUDE-ALS declined less than patients who received placebo. The trial showed effects favoring reldesemtiv across dose levels and timepoints with clinically meaningful magnitudes of effect observed at 12 weeks for the primary and secondary endpoints. The differences between reldesemtiv and placebo in SVC and ALSFRS-R total score observed after 12 weeks of treatment were still evident at follow-up, four weeks after the last dose of study drug.
The incidence of early treatment discontinuations, serious adverse events and clinical adverse events in FORTITUDE-ALS were similar between placebo and active treatment arms. The most common clinical adverse effects in the trial included fatigue, nausea and headache. The leading cause for early termination from FORTITUDE-ALS for patients who received placebo was progressive disease; the leading cause for early termination for patients who received reldesemtiv was a decline in cystatin C based estimated glomerular filtration rate (eGFR), a measure of renal function. Elevations in transaminases and declines in cystatin C eGFR were dose-related.
“Results from FORTITUDE-ALS are among the most impressive we have seen in a Phase 2 clinical trial in ALS,” said Dr. Shefner. “Especially noteworthy are the consistency and durability of effects observed across treatment arms on clinically meaningful endpoints.”
In collaboration with Astellas, Cytokinetics is developing reldesemtiv, a next-generation fast skeletal muscle troponin activator (FSTA), as a potential treatment for people living with debilitating diseases and conditions associated with skeletal muscle weakness and/or fatigue.
Primary Efficacy Endpoint and Additional Analyses
In FORTITUDE-ALS, the primary analysis of change from baseline to Week 12 in percent predicted SVC was analyzed using a mixed model for repeated measures (MMRM) with the contrast (-5, -1, 3, 3) to reflect the assumed weighted dose-response relationship for the placebo, 150 mg BID, 300 mg BID and 450 mg BID dose groups of reldesemtiv, respectively. While all doses of reldesemtiv demonstrated numerical reductions in SVC vs. placebo, the mixed model analysis was not statistically significant (p=0.11) (Figure 1). In a post-hoc analysis, when all active treatment groups were combined and compared to placebo, the trial showed a 27% reduction in the decline of SVC (p=0.10) (Figure 2).
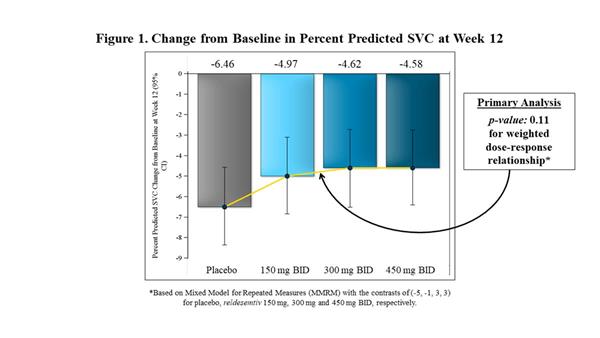 |
| Change from Baseline in Percent Predicted SVC at Week 12 |
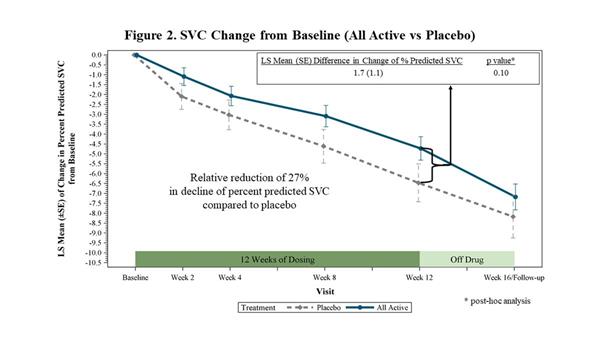 |
| SVC Change from Baseline (All Active vs Placebo) |
In FORTITUDE-ALS, the rate of decline in SVC in the placebo group was slower than has been observed in previous trials. FORTITUDE-ALS was powered with the expectation patients receiving placebo would decline approximately 8.0 percentage points during the 12-week trial. In FORTITUDE-ALS, patients receiving placebo declined 6.5 percentage points over 12 weeks.
Secondary Efficacy Endpoints: Additional Analyses
In a post-hoc analysis, FORTITUDE-ALS demonstrated a decrease in the decline of the ALSFRS-R from baseline to 12 weeks of 25% (p=0.01) when all active treatment groups combined were compared to placebo (Figure 3). The ALSFRS-R measures activities of daily living and has been deemed acceptable by regulatory authorities for approval and marketing authorization. According to a 2010 survey of ALS clinicians conducted by the
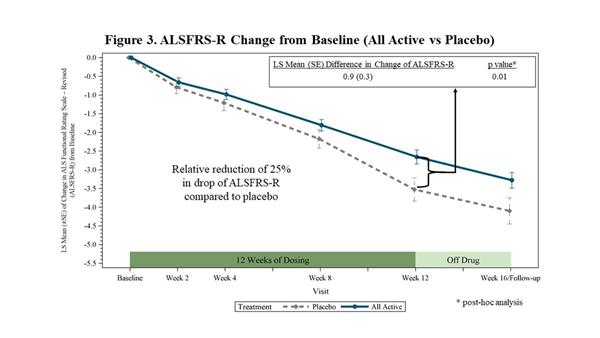 |
| ALSFRS-R Change from Baseline (All Active vs Placebo) |
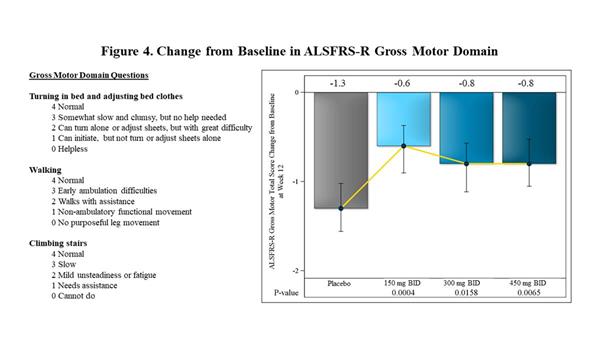 |
| Change from Baseline in ALSFRS-R Gross Motor Domain |
Within the ALSFRS-R domains, the largest effects observed at 12 weeks in FORTITUDE-ALS were in the Gross Motor Domain of the ALSFRS-R, which measures the ability of patients with ALS to turn in bed, walk and climb stairs. The effect of reldesemtiv for the Gross Motor Domain score was statistically significant at each dose vs. placebo (p=0.0004 for 150 mg BID, p=0.0158 for 300 mg BID, p=0.0065 for 450 mg BID) (Figure 4).
“While FORTITUDE-ALS did not meet the primary endpoint, we are encouraged by the results of the trial as they further validate the potential of skeletal muscle activation in treating patients battling ALS,” said Fady I. Malik, M.D., Ph.D., Cytokinetics’ Executive Vice President, Research and Development. “This Phase 2 trial of reldesemtiv demonstrated consistency of effect for doses, endpoints and timepoints and we believe the results support progression of reldesemtiv in further clinical trials toward potential registration.”
Cytokinetics Event/Conference Call/Webcast
Cytokinetics will host an investor event and conference call on
FORTITUDE-ALS: Clinical Trial Design
FORTITUDE-ALS is a Phase 2, double-blind, randomized, dose-ranging, placebo-controlled, parallel group study of reldesemtiv in patients with ALS. 458 eligible ALS patients from centers in the U.S., Canada, Europe and Australia were randomized (1:1:1:1) to receive either 150 mg, 300 mg or 450 mg of reldesemtiv or placebo dosed orally twice daily for 12 weeks. The primary efficacy endpoint was the change from baseline in the percent predicted SVC, a measure of respiratory function, at 12 weeks. Secondary endpoints included change from baseline in the ALS Functional Rating Scale – Revised (ALSFRS-R) and slope of the change from baseline in the mega-score of muscle strength measured by hand held dynamometry and handgrip dynamometry in patients on reldesemtiv; incidence and severity of treatment-emergent adverse events (TEAEs); and plasma concentrations of reldesemtiv at the sampled time points during the clinical trial.
In addition, exploratory endpoints were measured including the effect of reldesemtiv versus placebo on self-assessments of respiratory function made at home by the patient with assistance as needed by the caregiver; disease progression through quantitative measurement of speech production characteristics over time; disease progression through quantitative measurement of handwriting abilities over time; and change from baseline in quality of life (as measured by the ALSAQ-5) in patients on reldesemtiv.
About ALS
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that afflicts approximately 20,000 people in the United States and a comparable number of patients in Europe. Approximately 5,000 new cases of ALS are diagnosed each year in the United States. The average life expectancy of an ALS patient is approximately three to five years after diagnosis and only approximately 10 percent of patients survive for more than 10 years. Death is usually due to respiratory failure because of diminished strength in the skeletal muscles responsible for breathing. Few treatment options exist for these patients, resulting in a high unmet need for new therapies to address functional deficits and disease progression.
About Reldesemtiv
Skeletal muscle contractility is driven by the sarcomere, the fundamental unit of skeletal muscle contraction and a highly ordered cytoskeletal structure composed of several key proteins. Skeletal muscle myosin is the motor protein that converts chemical energy into mechanical force through its interaction with actin. A set of regulatory proteins, which includes tropomyosin and several types of troponin, make the actin-myosin interaction dependent on changes in intracellular calcium levels. Reldesemtiv, a next-generation FSTA arising from Cytokinetics’ skeletal muscle contractility program, slows the rate of calcium release from the regulatory troponin complex of fast skeletal muscle fibers, which sensitizes the sarcomere to calcium, leading to an increase in skeletal muscle contractility. Reldesemtiv has demonstrated pharmacological activity that may lead to new therapeutic options for diseases associated with muscle weakness and fatigue. In non-clinical models of spinal muscular atrophy (SMA), a skeletal muscle activator has demonstrated increases in submaximal skeletal muscle force and power in response to neuronal input and delays in the onset and reductions in the degree of muscle fatigue. Reldesemtiv has been the subject of five completed Phase 1 clinical trials in healthy volunteers, which evaluated the safety, tolerability, bioavailability, pharmacokinetics and pharmacodynamics of the drug candidate. Mid-stage clinical trials in patients with SMA, COPD and elderly adults with limited mobility have been completed. In the Phase 2 clinical study in patients with SMA, patients treated with reldesemtiv demonstrated increases in measures of endurance and stamina consistent with the mechanism of action.
About Cytokinetics and Astellas Collaboration
In 2013, Cytokinetics and Astellas formed a partnership focused on the research, development, and potential commercialization of skeletal muscle activators. The primary objective of the collaboration is to advance novel therapies for diseases and medical conditions associated with muscle impairment and weakness. Cytokinetics initially exclusively licensed to Astellas rights to co-develop and potentially co-commercialize reldesemtiv and other FSTAs in non-neuromuscular indications and to develop and commercialize other novel mechanism skeletal muscle activators in all indications. Under the agreement as subsequently expanded and amended, Astellas also has exclusive rights to co-develop and commercialize reldesemtiv and other FSTAs in certain neuromuscular indications (including SMA and ALS). Cytokinetics has certain development and commercialization rights, including the right to co-promote FSTAs for neuromuscular indications in the U.S., Canada and Europe and to co-promote the other collaboration products in the U.S. and Canada.
About Cytokinetics
Cytokinetics is a late-stage biopharmaceutical company focused on discovering, developing and commercializing first-in-class muscle activators and best-in-class muscle inhibitors as potential treatments for debilitating diseases in which muscle performance is compromised and/or declining. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact muscle function and contractility. Cytokinetics is collaborating with
For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on Twitter, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act's
Contact:
Cytokinetics
Diane Weiser
Vice President,
(415) 290-7757
__________________________________
1 Castrillo-Viguera, et al. Clinical significance in the change of decline in ALSFRS-R. Amyotrophic Lateral Sclerosis. 2010; 11: 178-180
Source: Cytokinetics, Incorporated




