Cytokinetics Announces Data From Phase 1 Study of CK-3773274 at the HFSA 23ʳᵈ Annual Scientific Meeting
Pharmacokinetic/Pharmacodynamic Relationship in Humans Similar to Preclinical Findings
Data Support Progression to Phase 2 Clinical Trial in Patients with Obstructive Hypertrophic Cardiomyopathy to Begin in Q4 2019
SOUTH SAN FRANCISCO, Calif.,
Phase 1 Design and Key Findings
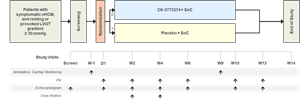
The primary objective of this Phase 1 double-blind, randomized, placebo-controlled, multi-part, single and multiple ascending dose study was to assess the safety and tolerability of CK-274 in healthy volunteers. The study design included single ascending dose cohorts and multiple ascending dose cohorts, with eight healthy subjects per cohort. Additional objectives included to describe the PK of CK-274 and its PD effects as measured by echocardiography, as well as to characterize the relationship between the two with regards to cardiac function. This study was not designed to identify a maximum tolerated dose of CK-274.
The study demonstrated that CK-274 was safe and well tolerated in healthy participants. No serious adverse events and no clinically meaningful changes in vital signs, ECGs or laboratory tests were observed. Stopping criteria for continued dose escalation were reached after a single dose of 75 mg and after 14 days of a daily of 10 mg dose. Decreases in ejection fraction below 50% were readily reversible within six hours following single doses and within 24-48 hours following 14 days of dosing. The pharmacokinetics of CK-274 were generally dose linear, and steady-state appeared evident within 14 days of dosing. Left ventricular ejection fraction decreased in an exposure dependent manner and the PK/PD relationship for CK-274 observed in humans was similar to that observed preclinically when adjusted for differences in protein binding. Specifically, the shallow exposure-response relationship observed preclinically appears to translate to humans and thereby may enable flexible dose optimization in humans.
“Our scientists originally pioneered this emerging area of muscle pharmacology and we have now advanced a potential next-in-class drug candidate that was optimized for its pharmacokinetic properties and therapeutic index, both of which are evident in this study,” said Fady I. Malik, MD, PhD, Cytokinetics’ Executive Vice President, Research and Development. “Importantly, steady state was achieved within two weeks of daily dosing and reversibility of drug effect within 24-48 hours following 14 days of dosing. These properties enable two-week dose titration in the planned Phase 2 trial and may translate to rapid onset, ease of titration and rapid symptom relief in the clinical setting.”
Planned Phase 2 Clinical Trial Design
Cytokinetics expects to begin a randomized, placebo-controlled Phase 2 trial of CK-274 in patients with symptomatic obstructive HCM later this year. The primary objective of the planned trial is to assess the safety and tolerability of CK-274 in these patients. Secondary objectives are to assess the PK and PD of CK-274 in these patents, guided by echocardiography, with two-week dose titration. CK-274 will be added to stable background medical therapy.
About CK-274 CK-274 is a novel, selective, oral, small molecule cardiac myosin inhibitor that company scientists discovered independent of its collaborations. CK-274 arose from an extensive chemical optimization program conducted with careful attention to therapeutic index and pharmacokinetic properties that may translate into next-in-class potential in clinical development. CK-274 was purposely designed to reduce the hypercontractility that is associated with hypertrophic cardiomyopathy (HCM). In preclinical models, CK-274 reduces myocardial contractility by binding directly to cardiac myosin at a distinct and selective allosteric binding site, thereby preventing myosin from entering a force producing state. CK-274 reduces the number of active actin-myosin cross bridges during each cardiac cycle and consequently reduces myocardial contractility. This mechanism of action may be therapeutically effective in conditions characterized by excessive hypercontractility, such as HCM. The preclinical pharmacokinetics of CK-274 were evaluated and optimized for potential rapid onset, ease of titration, individualized dosing and rapid symptom relief in the clinical setting. The initial focus of the development program for CK-274 will include an extensive characterization of its PK/PD relationship as has been a hallmark of Cytokinetics’ industry-leading development programs in muscle pharmacology. The overall development program will assess the potential of CK‑274 to improve exercise capacity and relieve symptoms in patients with hypercontractile ventricular function due to HCM. About Hypertrophic Cardiomyopathy Hypertrophic cardiomyopathy (HCM) is an inherited cardiovascular disorder in which the heart muscle (myocardium) becomes abnormally thick (hypertrophied). The thickening of cardiac muscle leads to the inside of the left ventricle becoming smaller and stiffer, and thus the ventricle becomes less able to relax and fill with blood. This ultimately limits the heart’s pumping function, resulting in symptoms including chest pain, dizziness, shortness of breath, or fainting during physical activity. A subset of patients with HCM are at high risk of progressive disease which can lead to ventricular arrhythmias, atrial fibrillation, stroke, heart failure and sudden cardiac death. There are no current medical treatments that directly address the hypercontractility that underlies HCM. About Cytokinetics Cytokinetics is a late-stage biopharmaceutical company focused on discovering, developing and commercializing first-in-class muscle activators and best-in-class muscle inhibitors as potential treatments for debilitating diseases in which muscle performance is compromised and/or declining. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact muscle function and contractility. Cytokinetics is collaborating with For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on Twitter, LinkedIn, Facebook and YouTube. Forward-Looking Statements This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act's Contact: A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5a6efc9d-d14e-4a7b-9ffe-4ab4de674521
Source: Cytokinetics, Incorporated |
|||||