Cytokinetics Announces Results From Cohort 3 of REDWOOD-HCM Presented at American College of Cardiology 71st Annual Scientific Session
Cohort 3 of REDWOOD-HCM enrolled thirteen patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract gradient (LVOT-G) of ≥50 mmHg whose background therapy included disopyramide and, in the majority (11 out of 13 patients), a beta-adrenergic blocker. These patients remained symptomatic despite use of disopyramide and represent a group of patients resistant to available medical therapies. All patients received up to three escalating doses of aficamten once daily (5, 10, 15 mg), titrated based on echocardiographic guidance. The doses of aficamten employed were the same as those used in Cohort 1 of REDWOOD-HCM. Overall treatment duration was 10 weeks with a 4-week follow up period after the last dose. All patients completed the study on treatment.
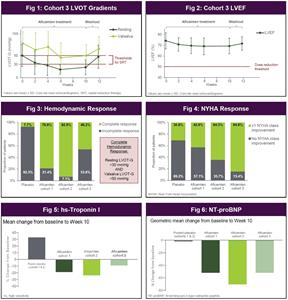
Patients in Cohort 3 demonstrated a substantial reduction in the mean (± SD) resting LVOT-G (from 50 ± 25 at baseline to 24 ± 17 mmHg at Week 10) and Valsalva LVOT-G (from 78 ± 27 to 50 ± 25 mmHg) (Figure 1). For the resting LVOT-G, the least square mean difference (± SE) for the change from baseline to Week 10 was -28 ± 3.2 mmHg (p < 0.0001) and for the Valsalva LVOT-G was -27 ± 5.9 mmHg (p = 0.0002). The relief of obstruction was accompanied by a modest reduction in left ventricular ejection fraction (LVEF) (from 74 ± 7% at baseline to 69 ± 7% at Week 10) (Figure 2). For LVEF, the least square mean difference (± SE) for the change from baseline to Week 10 was -4.8 ± 1.9% (p = 0.018). There were no patients who experienced a reduction in LVEF below the prespecified safety threshold of 50%.
Treatment with aficamten resulted in 6 of the 13 patients (46%) experiencing a complete hemodynamic response by Week 10 (Figure 3), with the remaining 7 (54%) still eligible for dose escalation to the highest dose of aficamten (20 mg) employed in SEQUOIA-HCM, the Phase 3 trial. Eleven of 13 patients (85%) experienced improvement in NYHA class by at least one class (Figure 4). In addition to hemodynamic and functional capacity improvements, patients also experienced a significant improvement in NT-proBNP and trended to lower hs-troponin I (Figures 5-6). The safety and tolerability of aficamten were consistent with prior experience in REDWOOD-HCM with no dose interruptions or treatment discontinuations and no serious adverse events. Coadministration of aficamten along with disopyramide and beta-blockers or calcium-channel blockers did not result in any significant electrocardiographic changes including in the QT-interval, or in blood pressure or heart rate.
“The results from Cohort 3 demonstrate that the addition of aficamten to standard of care therapy in the most treatment-resistant patients being treated with disopyramide results in safe, clinically meaningful improvements in LVOT gradients, functional class and cardiac biomarkers,” said Fady I. Malik, M.D., Ph.D., Cytokinetics’ Executive Vice President of Research & Development. “These results supported the inclusion of patients on background therapy with disopyramide in SEQUOIA-HCM, our ongoing Phase 3 clinical trial, designed to investigate whether aficamten can provide additional clinical benefit for these patients with no other alternative medical therapy.”
About REDWOOD-HCM
REDWOOD-HCM HCM (Randomized Evaluation of Dosing With CK-274 in Obstructive Outflow Disease in HCM) is a Phase 2, multi-center, randomized, placebo-controlled, double-blind, dose finding clinical trial of aficamten in patients with symptomatic obstructive HCM (oHCM). In Cohorts 1 and 2, patients continued taking background medications exclusive of disopyramide. Results from Cohorts 1 and 2 showed that treatment with aficamten for 10 weeks resulted in statistically significant reductions from baseline compared to placebo in the average resting left ventricular outflow tract pressure gradient (LVOT-G) and the average post-Valsalva LVOT-G. A large majority of patients treated with aficamten achieved the target goal of treatment, defined as resting gradient <30 mmHg and post-Valsalva gradient <50 mmHg at Week 10, compared to placebo. Patients treated with aficamten also saw improvements in heart failure symptoms and reductions in NT-proBNP, a biomarker of cardiac wall stress. Treatment with aficamten in REDWOOD-HCM was generally well tolerated and the incidence of adverse events on aficamten was similar to that of placebo. No serious adverse events were attributed to aficamten, and no treatment interruptions occurred on aficamten. Cohort 4 of REDWOOD-HCM is currently enrolling, in an open label fashion, patients with symptomatic non-obstructive HCM receiving background medical therapy. The primary objective is to determine the safety and tolerability of aficamten in patients with non-obstructive HCM.
About Aficamten
Aficamten is an investigational selective, small molecule cardiac myosin inhibitor discovered following an extensive chemical optimization program that was conducted with careful attention to therapeutic index and pharmacokinetic properties and as may translate into next-in-class potential in clinical development. Aficamten was designed to reduce the number of active actin-myosin cross bridges during each cardiac cycle and consequently suppress the myocardial hypercontractility that is associated with hypertrophic cardiomyopathy (HCM). In preclinical models, aficamten reduced myocardial contractility by binding directly to cardiac myosin at a distinct and selective allosteric binding site, thereby preventing myosin from entering a force producing state. The development program for aficamten is assessing its potential as a treatment that improves exercise capacity and relieves symptoms in patients with HCM as well as its long-term effects on cardiac structure and function. Aficamten received Breakthrough Therapy Designation for the treatment of symptomatic obstructive HCM from the
About Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a disease in which the heart muscle (myocardium) becomes abnormally thick (hypertrophied). The thickening of cardiac muscle leads to the inside of the left ventricle becoming smaller and stiffer, and thus the ventricle becomes less able to relax and fill with blood. This ultimately limits the heart’s pumping function, resulting in symptoms including chest pain, dizziness, shortness of breath, or fainting during physical activity. A subset of patients with HCM are at high risk of progressive disease which can lead to atrial fibrillation, stroke and death due to arrhythmias. There are no FDA approved medical treatments that directly address the hypercontractility that underlies HCM.
About
Cytokinetics is a late-stage biopharmaceutical company focused on discovering, developing and commercializing first-in-class muscle activators and next-in-class muscle inhibitors as potential treatments for debilitating diseases in which muscle performance is compromised. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact muscle function and contractility. Cytokinetics is readying for the potential commercialization of omecamtiv mecarbil, its cardiac muscle activator, following positive results from GALACTIC-HF, a large, international Phase 3 clinical trial in patients with heart failure. Cytokinetics is also developing aficamten, a next-generation cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, the Phase 3 clinical trial of aficamten in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). Aficamten is also being evaluated in non-obstructive HCM in Cohort 4 of the Phase 2 clinical trial, REDWOOD-HCM. Cytokinetics is also developing reldesemtiv, an investigational fast skeletal muscle troponin activator, currently the subject of COURAGE-ALS, a Phase 3 clinical trial in patients with amyotrophic lateral sclerosis (ALS). Cytokinetics continues its over 20-year history of pioneering innovation in muscle biology and related pharmacology focused to diseases of muscle dysfunction and conditions of muscle weakness.
For additional information about
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act").
CYTOKINETICS® and the
Contact:
Associate Director, Corporate Communications, Investor Relations
(650) 624-3283

Source: Cytokinetics, Incorporated